
Bazedoxifene (Conbriza®, Viviant®) is the first third-generation selective estrogen receptor modulator (SERM) and it is approved for the treatment of postmenopausal osteoporosis in the EU and Japan. Bazedoxifene contains an indole-based core binding domain that binds with high affinity to estrogen receptors and exhibits favourable effects on bone and lipid profiles, with no clinically relevant endometrial or breast stimulation. Oral bazedoxifene once daily reduced the incidence of new vertebral fractures in patients with postmenopausal osteoporosis in a large, well designed trial of 3 years' duration; both bazedoxifene and raloxifene were significantly more effective than placebo. Neither bazedoxifene nor raloxifene reduced the incidence of nonvertebral fractures in the overall study population; however, bazedoxifene, but not raloxifene, reduced the rate of nonvertebral fractures in high-risk patients. Moreover, data from patients who continued to receive the drug during a 2-year extension phase of this trial indicate that bazedoxifene continues to provide protection against new vertebral fractures for up to 5 years. Bazedoxifene also increases bone mineral density and reduces the levels of bone turnover markers. Bazedoxifene was generally well tolerated and did not detrimentally affect the reproductive tract or breast tissue in clinical trials, thereby demonstrating a favourable risk-benefit profile. A pharmacoeconomic analysis conducted from an EU perspective predicted bazedoxifene to be cost effective in some EU countries. Therefore, bazedoxifene presents another useful option for the treatment of postmenopausal osteoporosis, especially in those at high risk for osteoporotic fracture.
1. Introduction
Osteoporosis is a progressive, asymptomatic systemic skeletal disease characterized by low bone density and architectural deterioration of bone tissue.[1] The resulting loss in bone strength increases the risk of fractures, with hip and clinical vertebral fractures associated with particularly high morbidity and mortality as well as decreased quality of life.[1,2] An estimated 75 million people in the US, EU and Japan are thought to be affected by osteoporosis,[2] with these numbers expected to double over the next 50 years.[3] Furthermore, the economic burden associated with osteoporotic fracture is substantial, with estimated direct medical costs of $US17 billion in the US alone in 2005, and is expected to increase dramatically as the population ages.[4]
According to the WHO definition, osteoporosis can be diagnosed by estimating bone mineral density (BMD) and is defined as a BMD of 2.5 standard deviations or more below the mean for young healthy women (i.e. a T-score less than or equal to −2.5);[5] BMD measurements also provide prognostic information on the risk of fractures.[6] Osteoporosis is three times more common in women than in men, mainly due to differences in peak bone mass and menopause-related estrogen deficiency, which can lead to an accelerated rate of bone loss and decreased BMD.[2]
Fracture prevalence increases dramatically with age, with the risk doubling every 7–8 years beyond the age of 50 years.[1] This is of particular concern in Asian countries where the number of fractures is expected to increase in line with rapidly aging populations.[7-9] In addition to age, other risk factors for osteoporotic fracture include low body mass index (BMI), low BMD, family history of osteoporosis and previous fracture.[1]
The primary goal of postmenopausal osteoporosis management is to reduce fracture risk by slowing or stopping bone loss, increasing bone mass, improving bone quality and bone architecture, maintaining or increasing bone strength, and minimizing falls.[1,10] Antiresorptive agents are a major pharmacological treatment option for the prevention of osteoporotic fractures and include bisphosphonates (e.g. alendronate, ibandronate and risedronate) and selective estrogen receptor modulators (SERMs) [e.g. raloxifene].[2,6,11] While the therapeutic efficacy of these approaches in reducing fracture risk has been demonstrated,[6,12] their differing risk-benefit profiles mean that they must be appropriately tailored to specific patient populations.[13]
SERMs are structurally unique compounds that interact with estrogen receptors in target organs to confer agonist or antagonist effects, depending on the tissue type, making them an appealing option for the management of postmenopausal osteoporosis.[14] In clinical trials, the second-generation SERM raloxifene reduced the risk of vertebral, but not nonvertebral fracture and a slight stimulatory effect of the endometrium was observed.[15] More recently, the focus has been on the development of new SERMs to reduce vertebral and nonvertebral fracture risk, without stimulating breast or uterine tissue.
Bazedoxifene (Conbriza®, Viviant®) is a third-generation SERM approved for use in the EU[16] and Japan[17] for the treatment of postmenopausal osteoporosis. Although bazedoxifene in combination with conjugated estrogens (CEs) is also under development,[18] data pertaining to this combination are beyond the scope of this review. This article reviews the pharmacological, clinical efficacy and tolerability data relevant to the use of bazedoxifene in this indication.
2. Pharmacodynamic Properties
The pharmacodynamic properties of bazedoxifene have been determined in preclinical[19-22] and clinical[7,8,23-25] studies. Trial design details and data from the clinical trials are discussed in more detail in section 4.
2.1 Mechanism of Action
Ideally, a SERM for the treatment of postmenopausal osteoporosis would demonstrate agonist activity in bone, as well as the cardiovascular system and CNS, while eliciting neutral or antagonist activity in the endometrium and breast.[26] Although the precise mechanism of action of SERMs is unknown, they are thought to act through binding to estrogen receptors (ER)α and ERβ and, depending on the tissue, can exhibit agonist and/or antagonist properties.[14] This variation in activity is thought to be driven by differences in expression of ERα and ERβ, ER conformation upon ligand binding, and expression and binding of coregulator proteins.[14]
Bazedoxifene (figure 1) is an ER ligand containing an indole-based core binding domain, a point of difference compared with the benzothiophene core of raloxifene.[19] Bazedoxifene binds to both ERα and ERβ with high affinity, although it has a 4-fold higher affinity for ERα than for ERβ (concentration that produces 50% inhibition [IC50] 26 vs 99 nmol/L); its affinity for ERα is ≈10-fold lower than that of raloxifene (IC50 2.4 nmol/L).
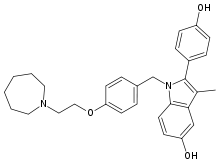
2.2 Preclinical Studies
In ovariectomized rat and monkey models of osteopenia, bazedoxifene demonstrated beneficial effects on skeletal profile parameters, including BMD, bone quality and compression strength.[19-22] In rats treated with bazedoxifene 0.3 or 3.0 mg/kg/day for 6 weeks, the skeleton was protected from bone loss. BMD in the proximal tibia was significantly (p ≤ 0.01) higher with bazedoxifene treatment than with control treatment and was comparable to that seen with raloxifene 3.0 mg/kg/day and the ER agonist estradiol 2 μg/day (table I).[19,21] In the same animals, compared with control treatment, a significant increase in compression strength of the fifth lumbar vertebra was observed with bazedoxifene but not with raloxifene (table I). The protective effects of bazedoxifene on BMD, bone mineral content, bone architecture and strength were observed up to 12 months.[20,21]
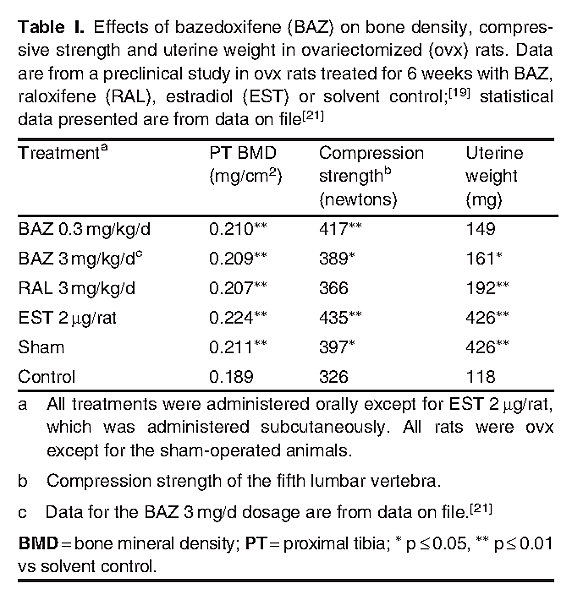
The beneficial effects of bazedoxifene on the skeleton of rats were observed with only mild stimulatory effects on the uterine endometrium and no apparent effect on the breast and CNS.[19,21] Small increases in uterine weight were observed with bazedoxifene (statistical significance [p ≤ 0.05] reached with bazedoxifene 3.0 mg/kg/day) relative to the control (table I), indicating bazedoxifene has limited uterine stimulatory effects.[19,21] In contrast, the uterine weight increase observed with raloxifene 3 mg/kg/day versus control had a statistical significance of p ≤ 0.01. In an in vitro study in the human breast cancer cell line MCF-7, bazedoxifene did not promote the proliferation of breast cancer cells and dose-dependently inhibited estradiol-stimulated breast cell proliferation.[19]
In a rat model of vasomotor instability, administration of bazedoxifene 0.3 mg/kg/day, a dosage that demonstrated beneficial effect on the bone (table I), had no effect on vasomotor changes (measured as thermoregulatory response), suggesting bazedoxifene does not have a detrimental effect on the CNS.[19]
2.3.2 Effects on the Endometrium and Breast Tissue
The effects of bazedoxifene on the endometrium and breast tissue have been evaluated in separate safety substudies[25,29-32] of phase II[25] and phase III[23,24] clinical trials. The effects of bazedoxifene on mammographic breast density have been evaluated in a retrospective ancillary study (n = 444)[33] of a 3-year phase III trial.[23]
Bazedoxifene therapy for 6 months to 5 years demonstrated neutral effects on endometrial and breast tissue.[7,8,24,25,29-33] For example, in a safety substudy (n = 753)[30] of an osteoporosis treatment trial,[23] changes from baseline in double-wall endometrial thickness with bazedoxifene 20 mg/day were not significantly different to those of raloxifene 60 mg/day or placebo at 24 months (−0.07 vs 0.16 and −0.08 mm), although a significant (p = 0.01) increase was seen with raloxifene compared with placebo (0.32 vs −0.11 mm) at the 12-month timepoint; the equivalent change with bazedoxifene was 0.11 mm. A low proportion of bazedoxifene and raloxifene recipients had endometrial thickness >5 mm at 24 months (2.3% and 3.5% [3.0% with placebo]).[30]
In the overall study population (n = 7492), low incidences (<1%) of endometrial hyperplasia, endometrial carcinoma and ovarian cysts were seen with bazedoxifene therapy, with no significant between-group differences observed across treatment groups.[30] Numerically fewer bazedoxifene recipients were diagnosed with endometrial polyps compared with raloxifene recipients (4 vs 7 and n = 3 for placebo).[30]
Bazedoxifene 20 mg/day therapy was not associated with an increase in the incidence of breast malignancy or abnormalities.[30] There were no statistically significant between-group differences in the incidence of breast carcinoma (0.3% with bazedoxifene vs 0.4% with raloxifene 60 mg/day vs 0.4% with placebo) or breast cysts (0.4% vs 0.9% vs 0.6%); however, the incidence of fibrocystic breast disease was significantly (p < 0.05) lower in bazedoxifene recipients than in raloxifene recipients (0.3% vs 0.8% [0.5% in placebo recipients]).[30] The incidences of breast pain with bazedoxifene, raloxifene and placebo treatments were 2.8%, 3.0% and 2.4%, respectively.[30]
In a retrospective, ancillary study of the largest phase III trial,[23] bazedoxifene had no effect on age-related changes in breast density in women with postmenopausal osteoporosis.[33] Mean changes in mammographic breast density from baseline with 24 months' bazedoxifene 20 mg/day therapy were small and were not significantly different to those of raloxifene or placebo (−1.2% vs −0.5% and −0.2%).[33]
Findings from the 2-year extension study[31] were consistent with those observed after 3 years.[23,30] Neutral effects of bazedoxifene therapy for 2 years on endometrial, ovarian and breast tissues were also observed in postmenopausal women at risk for osteoporosis.[29]
3. Pharmacokinetic Properties
The pharmacokinetic properties of bazedoxifene have been assessed in healthy postmenopausal women in clinical trials, with data available from a fully published study,[34,35] the Japanese prescribing information,[17] the EU summary of product characteristics[16] and the EU public assessment report for bazedoxifene.[22]
3.1 Absorption and Distribution
Oral bazedoxifene 20 mg was rapidly absorbed in healthy postmenopausal women (who were naturally menopausal or had undergone bilateral oophorectomy) [n = 23], with a time to maximum plasma concentration (tmax) of ≈2 hours (range ≈1–4 hours) and a mean oral absolute bioavailability of 6%.[22] After administration of multiple doses of once-daily bazedoxifene 20 mg, a mean maximum plasma concentration (Cmax) of 6.2 ng/mL and a mean area under the plasma concentration-time curve (AUC) of 82 ng · h/mL were reached.[16,22] Plasma concentrations of bazedoxifene increased in a linear fashion with single doses of between 0.5 and 120 mg, and multiple daily doses of between 1 and 80 mg.[16,17,22] With once-daily bazedoxifene dosing, steady-state concentrations of the drug were reached at the second week, with an accumulation of ≈2-fold.[22]
The absorption of bazedoxifene was influenced by food, although this was not considered to be clinically relevant.[22] Following administration of bazedoxifene 20 mg with a high-fat meal, Cmax and AUC were increased by 28% and 22%. Furthermore, in women who received multiple doses of bazedoxifene 20 mg, Cmax and AUC values at steady state were increased by 42% and 35% with a medium-fat meal.[16,17,22]
The mean volume of distribution of bazedoxifene was ≈15 L/kg following intravenous administration of a 3 mg dose and, in vitro, the drug is highly bound (>95%) to plasma proteins.[16,17,22]
3.2 Metabolism and Elimination
Bazedoxifene is extensively metabolized, with glucuronidation the predominant metabolic pathway.[34] The principal uridine diphosphate-glucuronosyl transferase (UGT) isoenzymes involved in the glucuronidation of bazedoxifene were UGT1A1, UGT1A8 and UGT1A10,[35] with little or no cytochrome P450 (CYP)-mediated metabolism observed.[17,34]
In healthy postmenopausal women (n = 6) after a single oral dose of radiolabelled bazedoxifene 20 mg, bazedoxifene-5-glucuronide was the major circulating metabolite (40–95%), while unchanged bazedoxifene (≤13%) and bazedoxifene-4-glucuronide (≤20%) were present at lower levels.[34] The major route of excretion of radiolabelled bazedoxifene was the faeces (≈85%); only ≈1% of the bazedoxifene dose was excreted in the urine.[34]
After intravenous administration of bazedoxifene, plasma clearance was 0.4 L/h/kg.[22] Apparent mean oral clearance of bazedoxifene normalized to a single 20 mg dose was 6.2 L/h/kg and at steady-state was 5.2 L/h/kg. The mean plasma elimination half-life (t½β) of bazedoxifene was ≈30 hours.[22]
3.4 Drug Interactions
In studies in healthy postmenopausal women, there were no clinically significant drug-drug interactions observed when bazedoxifene was administered with ibuprofen, azithromycin, atorvastatin, CEs or an antacid containing aluminium and magnesium hydroxide.[22]
Since bazedoxifene does not inhibit CYP enzymes in human liver microsomes, the probability of bazedoxifene interacting with other drugs that are metabolized by CYP enzymes is low.[22] Furthermore, when bazedoxifene is coadministered with warfarin, diazepam or digoxin, the potential for an interaction due to alterations of protein binding with bazedoxifene is low.[22]
4. Therapeutic Efficacy
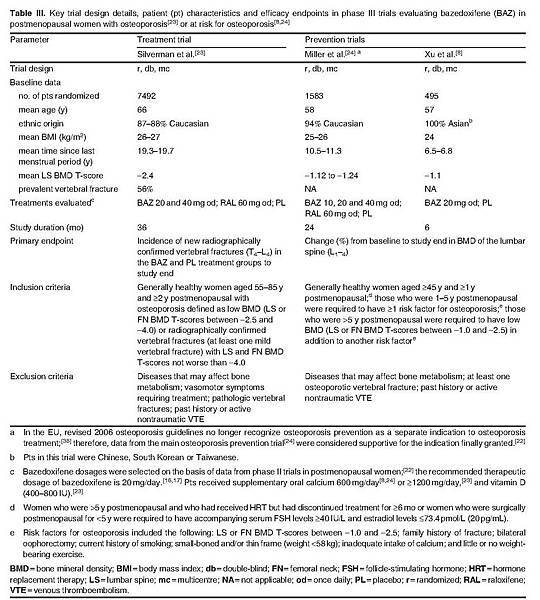
This section focuses on data from a large (n > 7000) placebo-controlled, phase III trial that compared the therapeutic efficacy of bazedoxifene 20 mg once daily with that of raloxifene 60 mg once daily in the treatment of osteoporosis in postmenopausal women.[23] Supportive data were obtained from phase III trials that evaluated the therapeutic efficacy of bazedoxifene in the prevention of postmenopausal osteoporosis in healthy women at risk for osteoporosis.[8,24] Key trial design details of the phase III studies are presented in table III. No statistically significant differences in patient demographic or baseline characteristics between treatment groups were reported.[8,23,24]
The efficacy of bazedoxifene in Japanese patients with postmenopausal osteoporosis was also evaluated in a randomized, double-blind, placebo-controlled, phase II study of 2 years' duration.[7] Patient eligibility criteria were generally similar to those of the phase III trials. The mean age was approximately 63 years and the mean BMI was approximately 21 kg/m2.[7]
Although three studies[7,23,24] included a treatment arm of bazedoxifene 40 mg/day, discussion in this section focuses on the recommended dosage of 20 mg/day that is approved in the EU and Japan.[16,17]
4.1 Effects on Fractures
4.1.1 Vertebral Fractures
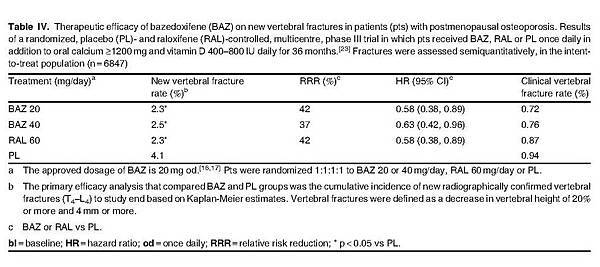
Bazedoxifene was effective in reducing the risk of new vertebral fracture in postmenopausal women with osteoporosis, according to the pivotal treatment trial.[23] Bazedoxifene 20 mg/day for 3 years resulted in significant reductions in the rate of new vertebral fracture compared with placebo in the intent-to-treat population (primary endpoint) [table IV], corresponding to an absolute rate reduction of 1.7%.[23] There was no statistically significant difference between bazedoxifene 20 mg/day and raloxifene 60 mg/day in terms of this endpoint.[23]
According to a subgroup analysis, baseline fracture status (prevalent fracture or no prevalent fracture at baseline) did not affect observed clinical outcomes with bazedoxifene therapy as indicated by a nonsignificant (p = 0.89) statistical interaction.[23] There was a low incidence (<1%) of clinical vertebral fractures observed in all groups, with no significant between-group differences (table IV).[23]
The therapeutic efficacy observed with bazedoxifene was sustained for up to 5 years, according to data from patients (n = 4216) who entered a 2-year extension phase[37] of the 3-year core study.[23] Based on Kaplan-Meier estimates, significant reductions in the rate of vertebral fracture were observed in bazedoxifene 20 and 40/20 mg/day recipients (patients received 40 mg/day for 4 years before transition to 20 mg/day for year 5 of the study) compared with placebo recipients after 5 years of therapy (4.5% and 3.9% vs 6.8%), corresponding to relative risk reductions (RRRs) of 35% (p = 0.014) and 40% (p = 0.005).[37]
4.2 Effects on Bone Mineral Density
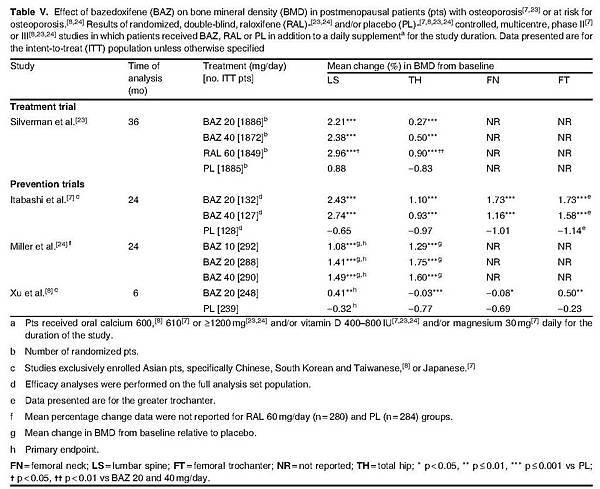
Bazedoxifene therapy was effective in the prevention of bone loss at various skeletal sites including the lumbar spine and total hip in postmenopausal women with osteoporosis or at risk for osteoporosis, with BMD significantly greater in bazedoxifene recipients than in placebo recipients after 6–36 months of treatment (table V).[7,8,23,24]
7. Dosage and Administration
Oral bazedoxifene 20 mg once daily is approved for the treatment of postmenopausal osteoporosis in Japan[17] and the treatment of postmenopausal osteoporosis in women at increased risk of fracture in the EU.[16] Bazedoxifene can be administered at any time of day, with or without food (EU).[16] Supplemental vitamin D and calcium should be added to the diet if daily intake is inadequate.[16,17]
Bazedoxifene is contraindicated in patients with active or a past history of VTE (including DVT, pulmonary embolism and retinal vein thrombosis) and in women of child-bearing potential in Japan and the EU[16,17] and in patients with antiphospholipid syndrome in Japan.[17] Bazedoxifene treatment should be discontinued in patients prior to prolonged immobilization (e.g. post-surgical recovery period, prolonged bed rest) and should be resumed only after the patient is fully ambulatory.[16,17]
Bazedoxifene should be administered with care in patients with a history of marked hypertriglyceridaemia as its use may increase serum triglyceride levels.[16,17] No studies with bazedoxifene have been undertaken in women with triglyceride levels >300 mg/dL (>3.4 mmol/L).[16,17] In Japan, bazedoxifene should be administered with care in patients with hepatic impairment,[17] while its use in this population is not recommended in the EU.[16]
The local manufacturer's prescribing information should be consulted for detailed information, including other contraindications, precautions, potential drug interactions and use in special patient populations.
8. Place of Bazedoxifene in the Treatment of Postmenopausal Osteoporosis
Osteoporotic fractures are associated with increased morbidity and mortality and loss in health-related quality of life, as well as causing a considerable economic burden.[1,2,6] Common fracture sites include the vertebrae, hip, distal forearm and proximal humerus, although hip and vertebrae are more commonly associated with acute pain and loss of function.[6] Vertebral fractures can often recur, with increasing disability with each subsequent fracture potentially leading to spinal deformity, while hip fractures nearly always require hospitalization, and lead to a rapid loss in physical and mental health and increased mortality.[2,6,9,11]
The primary goal of intervention in postmenopausal osteoporosis is to prevent bone loss, with the objective of reducing osteoporotic fracture risk.[6] Universal nonpharmacological recommendations to reduce fracture risk include adequate intake of calcium and vitamin D for all women aged 50 years and older, regular weight-bearing exercise, fall prevention, avoidance of tobacco use and excessive alcohol intake and ensuring a healthy bodyweight.[1,6,9,13,42]
Currently available pharmacological therapy for postmenopausal osteoporosis is based on modification of bone remodelling through inhibition of bone resorption (bisphosphonates [alendronate, ibandronate, risedronate and zoledronic acid], SERMs, monoclonal antibody [denosumab], calcitonin and HRT), stimulating bone formation (teriparatide, parathyroid hormone derivatives) or a combination of these effects (strontium ranelate).[6] However, availability of these agents varies greatly by country. For example, while calcitonin is available in many countries, it is used mainly in Japan and the US;[2] strontium ranelate is not available in the US or Japan;[1,7] and ibandronate, zoledronic acid and teriparatide are not approved in Japan.[7]
International guidelines generally advocate the use of agents that decrease both vertebral and nonvertebral fractures.[1,6,13] Of the available agents, bisphosphonates (generally available as once-daily, -weekly or -monthly oral formulations) are widely used.[1,6,42,43] Bisphosphonates have demonstrated more pronounced increases in BMD than SERMs in clinical trials; however, disadvantages of oral bisphosphonate formulations include potential gastrointestinal problems, and, due to their poor absorption, their administration procedures are complicated.[12,43] Furthermore, cases of osteonecrosis of the jaw and atypical femur fracture have been reported and may be related to over suppression of bone turnover with long-term therapy, although these are considered very rare and a causal link has not been established.[42,44] Denosumab, strontium ranelate and teriparatide also reduce both vertebral and nonvertebral fracture rates.[6] Denosumab provides a promising option for women at increased or high risk of fracture, although, as with bisphosphonates, concerns remain over the long-term effects of very low bone turnover seen with denosumab therapy, including the risk of osteonecrosis of the jaw.[45] Strontium ranelate, which is administered via oral suspension once daily at least 2 hours after food is associated with rare hypersensitivity syndromes and VTE, and is not currently available outside the EU.[6,46] Teriparatide (self-administered once daily via subcutaneous injection) use is often reserved for patients with severe osteoporosis.[47]
With the decrease in use of HRT in women with postmenopausal symptoms due to risk/benefit concerns with ongoing treatment (increase in heart disease, stroke and pulmonary embolism),[48] SERMs represent an attractive alternative option in postmenopausal women with osteoporosis, particularly in those who are unable to use bisphosphonates. To date, the second-generation SERM raloxifene has become a well established treatment option, although associated adverse events common to SERMs (VTEs, hot flushes, leg cramps) as well as the potential for endometrial stimulation may limit its use.[1,49] There is, therefore, a clear need for the development of new SERMs for the treatment of osteoporosis that have fewer adverse effects.
Bazedoxifene is a new, third-generation SERM indicated for the treatment of postmenopausal osteoporosis in Japan[17] and for postmenopausal osteoporosis in women at increased risk of fracture in the EU.[16] In the EU, approval was granted was on the basis of data reported in the pivotal osteoporosis treatment trial (section 4).[23]
The rapid absorption and long t½β (≈30 hours) of bazedoxifene allows for once-daily dosing, and the drug can be taken with or without food (section 3.1), which is preferable to oral bisphosphonates that have to be taken on an empty stomach with the patient then remaining upright for 30–60 minutes with no further food or liquid intake (other than water) permitted during this time.[12,43] Furthermore, no drug-drug interactions of clinical relevance have been reported with bazedoxifene (section 3.4).
Bazedoxifene therapy for 3 years was effective in reducing the incidence of new vertebral fractures in patients with postmenopausal osteoporosis (section 4.1.1) without causing detrimental effects on reproductive tract or breast tissue (section 2.3.2), with these benefits maintained for up to 5 years. The vertebral fracture risk with bazedoxifene did not differ from that with raloxifene, and the reduction in the risk of vertebral fracture was observed regardless of patient baseline fracture status (section 4.1.1).
Neither bazedoxifene nor raloxifene treatment reduced the risk of nonvertebral fractures in the overall population of postmenopausal women with osteoporosis in the head-to-head comparator trial (section 4.1.2). However, in high-risk patients (n = 1772), bazedoxifene significantly reduced the incidence of nonvertebral fracture compared with raloxifene or placebo, according to a post hoc analysis (section 4.1.3), with the number of new fractures significantly reduced with bazedoxifene versus placebo (from 17 vs 32 fractures [RRR 50%; p = 0.02]) and versus raloxifene (from 17 vs 30 fractures; [RRR 44%; p = 0.05]).[23] Nevertheless, post hoc evidence[50] from a large, placebo-controlled, phase III trial (Multiple Outcomes of Raloxifene Evaluation [MORE])[49] suggest that compared with placebo, raloxifene reduces the risk of nonvertebral fractures in patients with pre-existing severe vertebral fractures, although the number of patients was very limited (n = 393) as only patients with semiquantitative grade 3 fractures were included in the analysis. Furthermore, a reduction in all clinical fractures (composite of clinical spine, hip, forearm and humerus fracture) was seen with bazedoxifene in women at or above the FRAX®-based 10-year fracture probability threshold, with the magnitude of this effect increasing in line with increasing fracture probability (section 4.1.2); however, the post hoc nature of these analyses mean that these data should be interpreted with due caution. Further studies in women at high risk for osteoporotic fracture appear warranted.
Beneficial effects of bazedoxifene were also seen in terms of prevention of bone loss, with favourable changes in BMD observed at numerous skeletal sites in women with postmenopausal osteoporosis. The magnitude of this effect was generally greater for lumbar spine than for the total hip (section 4.2), possibly reflecting differences in fracture risk prevention at these sites. However, the effects of SERMs on BMD are relatively modest compared with other anti-resorptive therapies such as bisphosphonates and denosumab,[23,43,45] indicating that other factors may also contribute to the reduction in fracture risk seen with bazedoxifene, including reduction in bone turnover and bone material properties and/or microarchitecture, which may also strengthen bone.[23]
Consistent with pharmacokinetic studies in special patient populations (section 3.3), the efficacy of bazedoxifene in terms of improving BMD was observed irrespective of race, including Asian (Chinese, Korean and Taiwanese) and specifically Japanese patients (section 4.2). Although the effects of bazedoxifene on osteoporotic fracture were quantified in Japanese patients,[7] this trial was not powered to detect statistical changes for this endpoint; therefore, further larger studies in Asian populations may be necessary to fully evaluate the effects of bazedoxifene on osteoporotic fracture rate. The availability of effective pharmacological agents for the treatment postmenopausal osteoporosis is particularly important in Asia as the burden of osteoporosis is expected to grow rapidly as the population ages. In 2000, the number of Japanese individuals with osteoporosis was 11 million,[7] and ≈50% of all osteoporotic hip fractures are estimated to occur in Asia by the year 2050.[7-9]
Bazedoxifene was generally well tolerated in clinical trials, with a generally similar tolerability profile to that of raloxifene (section 5). Careful evaluation of the ‘class effects’ associated with SERMs, which include hot flushes, leg cramps and VTEs, was undertaken in bazedoxifene clinical trials, as well as the potential for stimulation of the endometrium or breast. Bazedoxifene was associated with a low incidence of VTEs (<1%), of which DVTs were the most common and were significantly higher with bazedoxifene and raloxifene therapy than with placebo (section 5). Bazedoxifene is contraindicated in patients with active or a past history of VTE (section 7). Further evaluation of VTE-related adverse events with bazedoxifene will be addressed in a post-marketing study.[22] Vasodilation (including hot flushes) and leg cramps were more frequent with bazedoxifene than with placebo, although most of these events were of mild to moderate severity (section 5).
While the overall tolerability profile of bazedoxifene was generally similar between the global and Asian studies (section 5), the reported incidence of hot flushes and leg cramps was considerably lower (≈3- and ≈9-fold) in the Asian studies (section 5.1). These results are consistent with evidence that suggests that Japanese and Chinese women may report fewer vasomotor symptoms because of differences in diet (low fat intake) and decreased estrogen variability compared with women of other races.[51] Asian women may also be less likely to report menopausal symptoms such as hot flushes due to cultural or linguistic differences.[52-54]
The uterus is thought to be the organ most sensitive to the ER antagonist/agonist activity of SERMs and, if stimulated, can lead to endometrial thickening, endometrial hyperplasia and malignancy.[55] The effects of bazedoxifene on the endometrium were negligible and appeared more favourable than with raloxifene, with fewer diagnoses of endometrial polyps and no changes in endometrial thickness with bazedoxifene, whereas significant increases were seen with raloxifene relative to placebo at 12 months (section 2.3.2). These data are in line with previous findings where raloxifene was associated with small but significant effects on endometrial thickness relative to placebo in the MORE trial.[49] Similarly, the SERM lasofoxifene, which is currently in clinical development, has been shown to reduce fractures, while simultaneously reducing breast cancer risk and having favourable effects on vaginal epithelium; however, it is associated with endometrial thickening, endometrial polyps and increased vaginal bleeding rates.[55] The apparent greater tissue-selective effects observed with bazedoxifene may, therefore, provide treatment advantages compared with other SERMs. Bazedoxifene therapy also had favourable effects on the lipid profile (section 2.3.3), consistent with raloxifene and lasofoxifene,[56,57] although further studies are required to determine the clinical relevance of this effect.
Importantly, there were study design differences between the osteoporosis treatment trial discussed in this review and previous osteoporosis treatment studies with raloxifene and risedronate that should be taken into account when comparing trial data.[23] Firstly, there was a lower proportion of patients in the placebo group in the bazedoxifene trial with new vertebral fractures (4%) than in placebo groups of previous studies with raloxifene (10%)[58] and risedronate (29%).[59] This is consistent with enrolment criteria in the bazedoxifene trial, which included patients who were at lower risk of fracture (section 4). Furthermore, patients in the bazedoxifene trial received a higher dosage of calcium supplementation than in previous trials of similar design (1200 mg/day vs 500–1000 mg/day), although this did not appear to mask the observed therapeutic effects of bazedoxifene.[23] A limitation of the clinical trials in Asian patients included the smaller patient populations evaluated (n < 500), which may have decreased the chances of observing clinically relevant efficacy and tolerability effects with bazedoxifene therapy.
While the data for bazedoxifene appear to indicate that it is effective and well tolerated, the longer-term effects of bazedoxifene therapy have yet to be determined. A double-blind, 4-year extension study (total study duration of 7 years)[60] of the pivotal osteoporosis treatment trial[23] is currently ongoing. Large head-to-head trials of bazedoxifene with other osteoporosis treatments such as bisphosphonates may help to further define the place of bazedoxifene in the treatment of postmenopausal osteoporosis, although logistic and economic barriers may limit the number of studies undertaken.[61,62]
A pharmacoeconomic analysis conducted from an EU perspective predicted bazedoxifene to be cost effective in some EU countries, with regard to the incremental cost per QALY gained, although the magnitude of the results varied widely between countries, with underlying fracture risk thought to be driving these differences (section 6).
To conclude, bazedoxifene is the first of the third-generation SERMs to be approved for the treatment of postmenopausal osteoporosis. Bazedoxifene therapy for up to 5 years was effective in reducing the incidence of new vertebral fractures in patients with postmenopausal osteoporosis. The reduction in vertebral fracture risk with bazedoxifene treatment did not significantly differ from that with raloxifene. Neither bazedoxifene nor raloxifene reduced the incidence of nonvertebral fractures overall; however, bazedoxifene, but not raloxifene, reduced the rate of nonvertebral fracture in high-risk patients. Bazedoxifene was generally well tolerated and did not detrimentally affect the reproductive tract or breast tissue, thereby demonstrating a favourable risk-benefit profile. Therefore, bazedoxifene presents another useful option for the treatment of postmenopausal osteoporosis, especially in those at high risk of osteoporotic fracture. Further clinical trials with bazedoxifene in high-risk patients, as well as longer-term studies, appear warranted to fully establish the place of bazedoxifene in the treatment of postmenopausal osteoporosis.
In the pivotal osteoporosis treatment trial,[23] significant improvements in lumbar spine and total hip BMD from baseline with bazedoxifene 20 mg/day relative to placebo were observed as early as 6 months (1.5% vs 0.5% and 0.8% vs 0.3%; data estimated from a graph) and were sustained throughout the study.[23] However, these improvements in lumbar spine and total hip BMD were greater with raloxifene 60 mg/day than with bazedoxifene (table V).[23]
As demonstrated in the treatment study, significant improvements in lumbar spine and total hip BMD from baseline were observed with bazedoxifene 20 mg/day relative to placebo within the first 6 months of therapy and were sustained throughout the large osteoporosis prevention study.[24]
Although lumbar spine BMD was significantly greater with bazedoxifene 20 mg/day than with placebo after 24 months of treatment (table V), lumbar spine BMD did not change significantly from baseline with bazedoxifene 20 mg/day, but did significantly (p < 0.001) decrease with placebo (numerical data not reported). The mean change in BMD from baseline at the lumbar spine with bazedoxifene 20 mg/day was not statistically different from that seen with raloxifene treatment (numerical data for raloxifene and placebo not reported) [table V].[24]
Furthermore, significantly greater BMD was observed in the total hip (table V), femoral neck (p < 0.01) and femoral trochanter (p < 0.001) with bazedoxifene 20 mg/day than with placebo, and values were not significantly different from those seen with raloxifene 60 mg/day (numerical data for femoral neck and trochanter not reported).[24]





 留言列表
留言列表
 線上藥物查詢
線上藥物查詢 